Typhoid fever
From Wikipedia, the free encyclopedia
| Typhoid fever |
| Classification and external resources |
 |
|---|
| Rose spots on the chest of a patient with typhoid fever due to the bacterium Salmonella typhi |
Typhoid fever, also known as enteric fever, Salmonella typhi or commonly just typhoid,[1] is an illness caused by the bacterium Salmonella enterica serovar typhi. Common worldwide, it is transmitted by the ingestion of food or water contaminated with feces from an infected person.[2] The bacteria then perforate through the intestinal wall and are phagocytosed by macrophages. Salmonella Typhi then alters its structure to resist destruction and allow them to exist within the macrophage. This renders them resistant to damage by PMN's, complement and the immune response. The organism is then spread via the lymphatics while inside the macrophages. This gives them access to the reticuloendothelial system and then to the different organs throughout the body. The organism is a Gram-negative short bacillus that is motile due to its peritrichous flagella. The bacteria grows best at 37 °C/99 °F – human body temperature.
Symptoms
Typhoid fever is characterized by a sustained fever as high as 40 °C (104 °F), profuse sweating, gastroenteritis, and nonbloody diarrhea. Less commonly a rash of flat, rose-colored spots may appear.[3]
Classically, the course of untreated typhoid fever is divided into four individual stages, each lasting approximately one week. In the first week, there is a slowly rising temperature with relative bradycardia, malaise, headache and cough. A bloody nose (epistaxis) is seen in a quarter of cases and abdominal pain is also possible. There is leukopenia, a decrease in the number of circulating white blood cells, with eosinopenia and relative lymphocytosis, a positive diazo reaction and blood cultures are positive for Salmonella Typhi or Paratyphi. The classic Widal test is negative in the first week.
In the second week of the infection, the patient lies prostrated with high fever in plateau around 40 °C (104 °F) and bradycardia (Sphygmo-thermic dissociation), classically with a dicrotic pulse wave. Delirium is frequent, frequently calm, but sometimes agitated. This delirium gives to typhoid the nickname of "nervous fever". Rose spots appear on the lower chest and abdomen in around 1/3 patients. There are rhonchi in lung bases. The abdomen is distended and painful in the right lower quadrant where borborygmi can be heard. Diarrhea can occur in this stage: six to eight stools in a day, green with a characteristic smell, comparable to pea-soup. However, constipation is also frequent. The spleen and liver are enlarged (hepatosplenomegaly) and tender and there is elevation of liver transaminases. The Widal reaction is strongly positive with antiO and antiH antibodies. Blood cultures are sometimes still positive at this stage.
In the third week of typhoid fever a number of complications can occur:
- Intestinal hemorrhage due to bleeding in congested Peyer's patches; this can be very serious but is usually non-fatal.
- Intestinal perforation in distal ileum: this is a very serious complication and is frequently fatal. It may occur without alarming symptoms until septicaemia or diffuse peritonitis sets in.
- Encephalitis
- Metastatic abscesses, cholecystitis, endocarditis and osteitis
The fever is still very high and oscillates very little over 24 hours. Dehydration ensues and the patient is delirious (typhoid state). By the end of third week the fever has started reducing (defervescence). This carries on into the fourth and final week.
Diagnosis
Diagnosis is made by any blood, bone marrow or stool cultures and with the Widal test (demonstration of salmonella antibodies against antigens O-somatic and H-flagellar). In epidemics and less wealthy countries, after excluding malaria, dysentery or pneumonia, a therapeutic trial time with chloramphenicol is generally undertaken while awaiting the results of Widal test and blood cultures.[4]
The term "enteric fever" is a collective terms that refers to typhoid and paratyphoid.[5]
Treatment
Where resistance is uncommon, the treatment of choice is a fluoroquinolone such as ciprofloxacin;[5][6] otherwise, a third-generation cephalosporin such as ceftriaxone or cefotaxime is the first choice.[7][8][9] Cefixime is a suitable oral alternative.[10][11]
Typhoid fever in most cases is not fatal. Antibiotics, such as ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole, Amoxicillin and ciprofloxacin, have been commonly used to treat typhoid fever in developed countries. Prompt treatment of the disease with antibiotics reduces the case-fatality rate to approximately 1%.
When untreated, typhoid fever persists for three weeks to a month. Death occurs in between 10% and 30% of untreated cases. Though in some communities case-fatality rates may be as high as 47%.
Resistance
Resistance to ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole and streptomycin is now common, and these agents have not been used as first line treatment now for almost 20 years. Typhoid that is resistant to these agents is known as multidrug-resistant typhoid (MDR typhoid).
Ciprofloxacin resistance is an increasing problem, especially in the Indian subcontinent and Southeast Asia. Many centres are therefore moving away from using ciprofloxacin as first line for treating suspected typhoid originating in South America, India, Pakistan, Bangladesh, Thailand or Vietnam. For these patients, the recommended first line treatment is ceftriaxone. It has also been suggested Azithromycin is better at treating typhoid in resistant populations than both fluoroquinolone drugs and ceftriaxone.[12] Azithromycin significantly reduces relapse rates compared with ceftriaxone.
There is a separate problem with laboratory testing for reduced susceptibility to ciprofloxacin: current recommendations are that isolates should be tested simultaneously against ciprofloxacin (CIP) and against nalidixic acid (NAL), and that isolates that are sensitive to both CIP and NAL should be reported as "sensitive to ciprofloxacin", but that isolates testing sensitive to CIP but not to NAL should be reported as "reduced sensitivity to ciprofloxacin". However, an analysis of 271 isolates showed that around 18% of isolates with a reduced susceptibility to ciprofloxacin (MIC 0.125–1.0 mg/l) would not be picked up by this method.[13] It is not certain how this problem can be solved, because most laboratories around the world (including the West) are dependent disc testing and cannot test for MICs.
| It has been suggested that Typhoid vaccine be merged into this article or section. (Discuss) |

Sanitation and hygiene are the critical measures that can be taken to prevent typhoid. Typhoid does not affect animals and therefore transmission is only from human to human. Typhoid can only spread in environments where human feces or urine are able to come into contact with food or drinking water. Careful food preparation and washing of hands are therefore crucial to preventing typhoid.
There are two vaccines currently recommended by the World Health Organization for the prevention of typhoid:[14] these are the live, oral Ty21a vaccine (sold as Vivotif Berna) and the injectable Typhoid polysaccharide vaccine (sold as Typhim Vi by Sanofi Pasteur and Typherix by GlaxoSmithKline). Both are between 50 to 80% protective and are recommended for travelers to areas where typhoid is endemic. There exists an older killed whole-cell vaccine that is still used in countries where the newer preparations are not available, but this vaccine is no longer recommended for use, because it has a higher rate of side effects (mainly pain and inflammation at the site of the injection).[14]
Transmission
Flying insects feeding on feces may occasionally transfer the bacteria through poor hygiene habits and public sanitation conditions. Public education campaigns encouraging people to wash their hands after defecating and before handling food are an important component in controlling spread of the disease. According to statistics from the United States Center for Disease Control, the chlorination of drinking water has led to dramatic decreases in the transmission of typhoid fever in the U.S.
A person may become an asymptomatic carrier of typhoid fever, suffering no symptoms, but capable of infecting others. According to the Centers for Disease Control approximately 5% of people who contract typhoid continue to carry the disease after they recover. The most famous asymptomatic carrier was Typhoid Mary. She was a young cook who was responsible for infecting about 47 people during her lifetime, killing three of the infected. This was the first time a perfectly healthy person was known to be responsible for an "epidemic".
Many carriers of typhoid were locked into an isolation ward never to be released in order to prevent further typhoid cases. These people often deteriorated mentally, driven mad by the conditions they lived in.[15]
With an estimated 16-33 million cases of annually resulting in 500,000 to 600,000 deaths in endemic areas, the World Health Organization identifies typhoid as a serious public health problem. Its incidence is highest in children and young adults between 5 and 19 years old.[16]
Heterozygous advantage
It is thought that cystic fibrosis may have risen to its present levels (1 in 1600 in UK) due to the heterozygous advantage that it confers against typhoid fever.[17] The CFTR protein is present in both the lungs and the intestinal epithelium, and the mutant cystic fibrosis form of the CFTR protein prevents entry of the typhoid bacterium into the body through the intestinal epithelium.
History
Around 430–426 B.C., a devastating plague, which some believe to have been typhoid fever, killed one third of the population of Athens, including their leader Pericles. The balance of power shifted from Athens to Sparta, ending the Golden Age of Pericles that had marked Athenian dominance in the ancient world. Ancient historian Thucydides also contracted the disease, but he survived to write about the plague. His writings are the primary source on this outbreak. The cause of the plague has long been disputed, with modern academics and medical scientists considering epidemic typhus the most likely cause. However, a 2006 study detected DNA sequences similar to those of the bacterium responsible for typhoid fever.[18] Other scientists have disputed the findings, citing serious methodologic flaws in the dental pulp-derived DNA study.[19] The disease is most commonly transmitted through poor hygiene habits and public sanitation conditions; during the period in question, the whole population of Attica was besieged within the Long Walls and lived in tents.
This fever received various names, such as gastric fever, abdominal typhus, infantile remittent fever, slow fever, nervous fever, pythogenic fever, etc. The name of " typhoid " was given by Louis in 1829, as a derivative from typhus.
In the late 19th century, typhoid fever mortality rate in Chicago averaged 65 per 100,000 people a year. The worst year was 1891, when the typhoid death rate was 174 per 100,000 persons.[20] The most notorious carrier of typhoid fever—but by no means the most destructive—was Mary Mallon, also known as Typhoid Mary. In 1907, she became the first American carrier to be identified and traced. She was a cook in New York; some believe she was the source of infection for several hundred people. She is closely associated with forty-seven cases and three deaths.[21] Public health authorities told Mary to give up working as a cook or have her gall bladder removed. Mary quit her job but returned later under a false name. She was detained and quarantined after another typhoid outbreak. She died of pneumonia after 26 years in quarantine.
In 1897, Almroth Edward Wright developed an effective vaccine. In 1909, Frederick F. Russell, a U.S. Army physician, developed an American typhoid vaccine and two years later his vaccination program became the first in which an entire army was immunized. It eliminated typhoid as a significant cause of morbidity and mortality in the U.S. military.
Most developed countries saw declining rates of typhoid fever throughout first half of 20th century due to vaccinations and advances in public sanitation and hygiene. Antibiotics were introduced in clinical practice in 1942, greatly reducing mortality. Today, incidence of typhoid fever in developed countries is around 5 cases per 1,000,000 people per year.
An outbreak in the Democratic Republic of Congo in 2004-05 recorded more than 42,000 cases and 214 deaths.[16]
Typhoid fever was also known as suette milliaire in nineteenth-century France.
Famous typhoid victims
|
|
|
Famous people who have had the disease include:
- Abigail Adams, wife of former United States President John Adams
- Albert of Saxe-Coburg-Gotha, British prince consort, Queen Victoria's husband
- Jean Baudrillard, cultural theorist, sociologist and philosopher
- Arnold Bennett, novelist
- Belle Boyd, female confederate spy
- Gonville Bromhead, Victoria Cross recipient for actions during Battle of Rorke's Drift
- John Buford
- Louisa May Alcott, author of Little Women records contracting it in Hospital Sketches
- Charles Darwin, naturalist, during his visit to Chile with HMS Beagle in 1835
- Stephen A. Douglas, United States politician
- Alexander Alexandrovich Friedman
- Mark Hanna, United States politician
- Gerard Manley Hopkins, English poet
- Archduke Karl Ludwig of Austria
- Mary Henrietta Kingsley
- William Wallace Lincoln, son of Abraham Lincoln
- Joseph Lucas
- James Martin (Australian soldier), Youngest known ANZAC
- Frank McCourt, contracted typhoid fever during his childhood, but survived
- Alice Hathaway Lee Roosevelt, wife of United States President Theodore Roosevelt
- Martha Bulloch Roosevelt, mother of Theodore Roosevelt
- Franz Schubert, composer
- William Tecumseh Sherman, Jr., son of General William T Sherman
- Joseph Smith Jr., first Prophet of the Church of Jesus Christ of Latter Day Saints (also known as Mormons), contracted typhoid fever during childhood (7 years old), but survived
- Leland Stanford, Jr.
- Henry Frederick Stuart, Prince of Wales, original heir to the throne of James I of England
- Thomas Vincent Welch
- Wilbur Wright
In medicine, diarrhea (from the Greek, "diarrhoia" meaning "a flowing through"[1]), also spelled diarrhoea (see spelling differences), is the condition of having frequent loose or liquid bowel movements. Acute diarrhea is a common cause of death in developing countries and the second most common cause of infant deaths worldwide. The loss of fluids through diarrhea can cause severe dehydration which is one cause of death in diarrhea sufferers. Along with water, sufferers also lose dangerous amounts of important salts, electrolytes, and other nutrients.
Causes

Diarrhea commonly results from gastroenteritis caused by viral infections, parasites or bacterial toxins.[2] In sanitary living conditions where there is ample food and a supply of clean water, an otherwise healthy patient usually recovers from viral infections in a few days. However, for ill or malnourished individuals diarrhea can lead to severe dehydration and can become life-threatening without treatment.[3]
Diarrhea can also be a symptom of more serious diseases, such as dysentery, cholera, or botulism, and can also be indicative of a chronic syndrome such as Crohn's disease or severe mushroom poisoning syndromes. Though appendicitis patients do not generally have violent diarrhea, it is a common symptom of a ruptured appendix. It is also an effect of severe radiation sickness.
Diarrhea can also be caused by dairy intake in those who are lactose intolerant.
Symptomatic treatment for diarrhea involves the patient consuming adequate amounts of water to replace that loss, preferably mixed with electrolytes to provide essential salts and some amount of nutrients. For many people, further treatment is unnecessary. The following types of diarrhea indicate medical supervision is required:
- Diarrhea in infants
- Moderate or severe diarrhea in young children;
- Diarrhea associated with blood
- Diarrhea that continues for more than two days;
- Diarrhea that is associated with more general illness such as non-cramping abdominal pain, fever, weight loss, etc;
- Diarrhea in travelers, since they are more likely to have exotic infections such as parasites;
- Diarrhea in food handlers, because of the potential to infect others;
- Diarrhea in institutions such as hospitals, child care centers, or geriatric and convalescent homes.
A severity score is used to aid diagnosis in children.[4]
Types of diarrhea
There are at least four types of diarrhea: secretory diarrhea, osmotic diarrhea, motility-related diarrhea, and inflammatory diarrhea.
Secretory diarrhea
Secretory diarrhea means that there is an increase in the active secretion, or there is an inhibition of absorption. There is little to no structural damage. The most common cause of this type of diarrhea is a cholera toxin that stimulates the secretion of anions, especially chloride ions. Therefore, to maintain a charge balance in the lumen, sodium is carried with it, along with water.
Osmotic diarrhea
Osmotic diarrhea occurs when too much water is drawn into the bowels. This can be the result of maldigestion (e.g., pancreatic disease or Coeliac disease), in which the nutrients are left in the lumen to pull in water. Osmotic diarrhea can also be caused by osmotic laxatives (which work to alleviate constipation by drawing water into the bowels). In healthy individuals, too much magnesium or vitamin C or undigested lactose can produce osmotic diarrhea and distention of the bowel. A person who does not have lactose intolerance can have difficulty absorbing lactose after an extraordinarily high intake of dairy products. In persons who do not have fructose malabsorption, excess fructose intake can still cause diarrhea. High-fructose foods that also have a high glucose content are more absorbable and less likely to cause diarrhea. Sugar alcohols such as sorbitol (often found in sugar-free foods) are difficult for the body to absorb and, in large amounts, may lead to osmotic diarrhea.
Motility-related diarrhea
Motility-related diarrhea is caused by the rapid movement of food through the intestines (hypermotility). If the food moves too quickly through the GI tract, there is not enough time for sufficient nutrients and water to be absorbed. This can be due to a vagotomy or diabetic neuropathy, or a complication of menstruation. Hyperthyroidism can produce hypermotility and lead to pseudodiarrhea and occasionally real diarrhea. Diarrhea can be treated with antimotility agents (such as loperamide).
Inflammatory diarrhea
Inflammatory diarrhea occurs when there is damage to the mucosal lining or brush border, which leads to a passive loss of protein-rich fluids, and a decreased ability to absorb these lost fluids. Features of all three of the other types of diarrhea can be found in this type of diarrhea. It can be caused by bacterial infections, viral infections, parasitic infections, or autoimmune problems such as inflammatory bowel diseases. It can also be caused by tuberculosis, colon cancer, and enteritis.
Dysentery
Generally, if there is blood visible in the stools, it is not diarrhea, but dysentery. The blood is trace of an invasion of bowel tissue. Dysentery is caused by an excess of water by a release of antidiuretic hormone from the posterior pituitary gland. Dysentery is a symptom of, among others, Shigella, Entamoeba histolytica, and Salmonella.
Infectious diarrhea
Infectious diarrhea is diarrhea caused by a microbe such as a bacterium, parasite, or virus.
Malabsorption
Malabsorption is the inability to absorb food, mostly in the small bowel but also due to the pancreas.
Causes include celiac disease (intolerance to wheat, rye, and barley gluten, the protein of the grain), lactose intolerance (intolerance to milk sugar, common in non-Europeans), fructose malabsorption, pernicious anemia (impaired bowel function due to the inability to absorb vitamin B12), loss of pancreatic secretions (may be due to cystic fibrosis or pancreatitis), short bowel syndrome (surgically removed bowel), radiation fibrosis (usually following cancer treatment), and other drugs, including agents used in chemotherapy.
Inflammatory bowel disease
The two overlapping types here are of unknown origin:
- Ulcerative colitis is marked by chronic bloody diarrhea and inflammation mostly affects the distal colon near the rectum.
- Crohn's disease typically affects fairly well demarcated segments of bowel in the colon and often affects the end of the small bowel.
Irritable Bowel Syndrome
Another possible cause of diarrhea is Irritable Bowel Syndrome (IBS). Symptoms defining IBS: abdominal discomfort or pain relieved by defecation and unusual stool (diarrhea or constipation or both) or stool frequency, for at least 3 days a week over the previous 3 months.[5] IBS symptoms can be present in patients with a variety of conditions including food allergies, infective diarrhea, celiac, and inflammatory bowel diseases. Treating the underlying condition (celiac disease, food allergy, bacterial dysbiosis, etc.) usually resolves the diarrhea.[6] IBS can cause visceral hypersensitivity. While there is no direct treatment for undifferentiated IBS, symptoms, including diarrhea, can sometimes be managed through a combination of dietary changes, soluble fiber supplements, and/or medications.
It is important to note that IBS can often be confused with Giardiasis since false negative tests for giardia can result in a misdiagnoses of the actual cause, a parasitic infection.[7]
Other important causes
- Ischemic bowel disease. This usually affects older people and can be due to blocked arteries.
- Bowel cancer: Some (but not all) bowel cancers may have associated diarrhea. Cancer of the large intestine is most common.
- Hormone-secreting tumors: some hormones (e.g. serotonin) can cause diarrhea if excreted in excess (usually from a tumor).
- Bile salt diarrhea: excess bile salt entering the colon rather than being absorbed at the end of the small intestine can cause diarrhea, typically shortly after eating. Bile salt diarrhea is a bad side-effect of gallbladder removal. It is usually treated with cholestyramine, a bile acid sequestrant.
- Celiac Disease
- Intestinal protozoa such as Giardiasis[7]
Alcohol
Chronic diarrhea can be caused by chronic ethanol ingestion.[8] Consumption of alcohol affects the body's capability to absorb water - this is often a symptom that accompanies a hangover after a binge drinking session. The alcohol itself is absorbed in the intestines and as the intestinal cells absorb it, the toxicity causes these cells to lose their ability to absorb water. This leads to an outpouring of fluid from the intestinal lining, which is in turn poorly absorbed. The diarrhea usually lasts for several hours until the alcohol is detoxified and removed from the digestive system. Symptoms range from person to person and are influenced by both the amount consumed as well as physiological differences.
Treatment
In many cases of diarrhea, replacing lost fluid and salts is the only treatment needed. This is usually by mouth – oral rehydration therapy – or, in very severe cases, intravenously.
Diet restriction such as limiting milk has no effect on the duration of diarrhea.[9] Medicines such loperamide (Imodium), bismuth subsalicylate (as found in Pepto Bismol and Kaopectate) may be beneficial, however they may be contraindicated in certain situations.[10] Prescribed medications sometimes contain pain-killers, such as morphine or codeine, to counter the cramps that can accompany diarrhea.
Evolutionary medicine
According to two researchers into evolutionary medicine, Nesse and Williams,[11] diarrhea functions as an evolved expulsion defense mechanism. As a result, if it is stopped, there might be a delay in illness recovery. They cite in support of this argument research carried out by DuPont and Hornick that was published in the Journal of the American Medical Association (JAMA)[12] showed that treating Shigella with the anti-diarrhea drug (Lomotil) caused people to stay feverish twice as long as those not so treated. The researchers indeed themselves observed that: "Lomotil may be contraindicated in shigellosis. Diarrhea may represent a defense mechanism".
| Constipation | |
| Classification and external resources | |
 | |
|---|---|
| Constipation in a young child as seen by X-ray | |
| ICD-10 | K59.0 |
| ICD-9 | 564.0 |
| DiseasesDB | 3080 |
| MedlinePlus | 003125 |
| eMedicine | med/2833 |
| MeSH | D003248 |
Constipation, costiveness, or irregularity is a condition of the digestive system in which a person (or animal) experiences hard feces (faeces) that are difficult to expel. This usually happens because the colon absorbs too much water from the food. If the food moves through the gastro-intestinal tract too slowly, the colon may absorb too much water, resulting in feces that are dry and hard. Defecation may be extremely painful, and in severe cases (fecal impaction) lead to symptoms of bowel obstruction. The term obstipation is used for severe constipation that prevents passage of both stools and gas. Causes of constipation may be dietary, hormonal, anatomical, a side effect of medications (e.g. some opiates), or an illness or disorder. Treatments consist of changes in dietary and exercise habits, the use of laxatives, and other medical interventions depending on the underlying cause.
Signs and symptoms

In common constipation, the stool is hard, difficult, and painful to pass. Usually, there is an infrequent urge to void. Straining to pass stool may cause hemorrhoids. In later stages of constipation, the abdomen may become distended and diffusely tender and cramp, occasionally with enhanced bowel sounds.
The definition of constipation includes the following:[1]
- infrequent bowel movements (typically three times or fewer per week)
- difficulty during defecation (straining during more than 25% of bowel movements or a subjective sensation of hard stools), or
- the sensation of incomplete bowel evacuation.
Severe cases ("fecal impaction") may feature symptoms of bowel obstruction (vomiting, very tender abdomen) and "paradoxical diarrhea", where soft stool from the small intestine bypasses the impacted matter in the colon.
Diagnosis
The diagnosis is essentially made from the patient's description of the symptoms. Bowel movements that are difficult to pass, very firm, or made up of small pellets (such as those excreted by rabbits) qualify as constipation, even if they occur every day. Other symptoms related to constipation can include bloating, distension, abdominal pain, or a sense of incomplete emptying.[2]
Inquiring about dietary habits may reveal a low intake of dietary fiber or inadequate amounts of fluids. Constipation as a result of poor ambulation or immobility should be considered in the elderly. Constipation may arise as a side effect of medications, including antidepressants, which can suppress acetylcholine[3][4] and opiates, which can slow the movement of food through the intestines[5]. Rarely, other symptoms suggestive of hypothyroidism may be elicited.[citation needed]
During physical examination, scybala (manually palpable lumps of stool) may be detected on palpation of the abdomen. Rectal examination gives an impression of the anal sphincter tone and whether the lower rectum contains any feces or not; if so, then suppositories or enemas may be considered. Otherwise, oral medication may be required. Rectal examination also gives information on the consistency of the stool, presence of hemorrhoids, admixture of blood and whether any tumors or abnormalities are present.
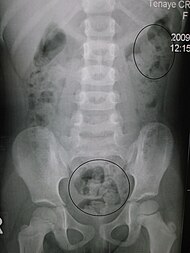
X-rays of the abdomen, generally only performed on hospitalized patients or if bowel obstruction is suspected, may reveal impacted fecal matter in the colon, and confirm or rule out other causes of similar symptoms.
Chronic constipation (symptoms present for more than 3 months at least 3 days per month) associated with abdominal discomfort is often diagnosed as irritable bowel syndrome (IBS) when no obvious cause is found. Physicians caring for patients with chronic constipation are advised to rule out obvious causes through normal testing.[6]
Colonic propagating pressure wave sequences (PSs) are responsible for discrete movements of content and are vital for normal defecation. Deficiencies in PS frequency, amplitude and extent of propagation are all implicated in severe defecatory dysfunction. Mechanisms that can normalise these aberrant motor patterns may help rectify the problem. Recently the novel therapy of sacral nerve stimulation (SNS) has been utilized for the treatment of severe constipation. [7]
Causes
The main causes of constipation include:
- Hardening of the feces
- Insufficient intake of dietary fiber
- Dehydration from any cause or inadequate fluid intake
- Medication, e.g. diuretics and those containing iron, calcium, aluminum
- Paralysis or slowed transit, where peristaltic action is diminished or absent, so that feces are not moved along
- Hypothyroidism (underactive thyroid gland)
- Hypokalemia
- Injured anal sphincter (patulous anus)
- Medications, such as loperamide, opioids (e.g. codeine & morphine) and certain tricyclic antidepressants
- Severe illness due to other causes
- Acute porphyria (a rare inherited condition)
- Lead poisoning
- Lactose Intolerance
- Dyschezia (usually the result of suppressing defecation)
- Diverticula
- Tumors, either of the bowel or surrounding tissues
- Obstructed defecation, due to:
- Mechanical causes from morphological abnormalities of the anorectum including megarectum, rectal prolapse, rectocele, and enterocele
- Functional causes from neurological disorders and dysfunction of the pelvic floor muscles or anorectal muscles, including anismus, descending perineum syndrome, and Hirschsprung's disease
- Retained foreign body or a bezoar
- Psychosomatic constipation, based on anxiety or unfamiliarity with surroundings.
- Functional constipation
- Constipation-predominant irritable bowel syndrome, characterized by a combination of constipation and abdominal discomfort and/or pain[8]
- Smoking cessation (nicotine has a laxative effect)[9]
- Abdominal surgery, other types of surgery, childbirth
- Severe dehydration
Treatment
In people without medical problems, the main intervention is to increase the intake of fluids (preferably water) and dietary fiber. The latter may be achieved by consuming more vegetables and fruit and whole meal bread, and pulses such as baked beans and chick peas and by adding linseeds to one's diet. The routine non-medical use of laxatives is to be discouraged as this may result in bowel action becoming dependent upon their use. Enemas can be used to provide a form of mechanical stimulation. However, enemas are generally useful only for stool in the rectum, not in the intestinal tract.
Lactulose, a nonabsorbable synthetic sugar that keeps sodium and water inside the intestinal lumen, relieves constipation. It can be used for months together. Among the other safe remedies, fiber supplements, lactitiol, sorbitol, milk of magnesia, lubricants etc. may be of value. Electrolyte imbalance e.g. Hyponatremia may occur in some cases especially in diabetics.
In alternative and traditional medicine, colonic irrigation, enemas, exercise, diet, and herbs are used to treat constipation. The mechanism of the herbal, enema, and colonic irrigation treatments often includes the breakdown of impacted and hardened fecal matter.
Laxatives
Laxatives may be necessary in people in whom dietary or other interventions are not effective or are inappropriate. Laxatives should be used with caution and only as necessary. The following sequence of laxative use is recommended: bulk forming, then stool softeners, then osmotic, then stimulants, then suppositories, and finally enemas (only as a last resort). The reason for this cautious use is because laxatives can lead to dependence, and like all medications they have side effects. Laxatives should not be used if there are signs and/or symptoms of a bowel obstruction. [10]
Physical intervention
Constipation that resists all the above measures requires physical intervention. Manual dissimpaction (the physical removal of impacted stool) is done for those patients who have lost control of their bowels secondary to spinal injuries. Manual dissimpaction is also used by physicians and nurses to relieve rectal impactions. Finally, manual dissimpaction can occasionally be done under sedation or a general anesthetic—this avoids pain and loosens the anal sphincter.
Many of the products are widely available over-the-counter. Enemas (clysters) are a remedy occasionally used for hospitalized patients in whom the constipation has proven to be severe, dangerous in other ways, or resistant to laxatives. Sorbitol, glycerin and arachis oil suppositories can be used. Severe cases may require phosphate solutions introduced as enemas.
Paediatrics/Pediatrics
Lactulose and milk of magnesia has been compared to PEG (polyethylene glycol) in children. They had similar side effects but PEG was more effective at treating constipation.[11][12] Osmotic laxatives are recommended over stimulant laxatives.[13]
Prevention
Constipation is usually easier to prevent than to treat. The relief of constipation with osmotic agents, i.e. lactulose, polyethylene glycol (PEG), or magnesium salts, should immediately be followed with prevention using increased fibre (fruits, vegetables, and grains) and a nightly decreasing dose of osmotic laxative. With continuing narcotic use, for instance, nightly doses of osmotic agents can be given indefinitely (without harm) to cause a daily bowel movement.
Recent controlled studies have questioned the role of physical exercise in the prevention and management of chronic constipation, while exercise is often recommended by published materials on the subject.[14]
In various conditions (such as the use of codeine or morphine), combinations of hydrating (e.g. lactulose or glycols), bulk-forming (e.g. psyllium) and stimulant agents may be necessary to prevent constipation.
The adult male worm is unimportant in the pathogenesis, since it dies after copulation.
What is appendicitis?
Appendicitis is a painful swelling and infection of the appendix.
[Top]
What is the appendix?
The appendix is a fingerlike pouch attached to the large intestine and located in the lower right area of the abdomen. Scientists are not sure what the appendix does, if anything, but removing it does not appear to affect a person’s health. The inside of the appendix is called the appendiceal lumen. Mucus created by the appendix travels through the appendiceal lumen and empties into the large intestine.

The appendix is a fingerlike pouch attached to the large intestine in the lower right area of the abdomen.
[Top]
What causes appendicitis?
Obstruction of the appendiceal lumen causes appendicitis. Mucus backs up in the appendiceal lumen, causing bacteria that normally live inside the appendix to multiply. As a result, the appendix swells and becomes infected. Sources of obstruction include
- feces, parasites, or growths that clog the appendiceal lumen
- enlarged lymph tissue in the wall of the appendix, caused by infection in the gastrointestinal tract or elsewhere in the body
- inflammatory bowel disease, including Crohn’s disease and ulcerative colitis
- trauma to the abdomen
An inflamed appendix will likely burst if not removed. Bursting spreads infection throughout the abdomen—a potentially dangerous condition called peritonitis.
[Top]
Who gets appendicitis?
Anyone can get appendicitis, but it is more common among people 10 to 30 years old. Appendicitis leads to more emergency abdominal surgeries than any other cause.
[Top]
What are the symptoms of appendicitis?
Most people with appendicitis have classic symptoms that a doctor can easily identify. The main symptom of appendicitis is abdominal pain.
The abdominal pain usually
- occurs suddenly, often causing a person to wake up at night
- occurs before other symptoms
- begins near the belly button and then moves lower and to the right
- is new and unlike any pain felt before
- gets worse in a matter of hours
- gets worse when moving around, taking deep breaths, coughing, or sneezing
Other symptoms of appendicitis may include
- loss of appetite
- nausea
- vomiting
- constipation or diarrhea
- inability to pass gas
- a low-grade fever that follows other symptoms
- abdominal swelling
- the feeling that passing stool will relieve discomfort
Symptoms vary and can mimic other sources of abdominal pain, including
- intestinal obstruction
- inflammatory bowel disease
- pelvic inflammatory disease and other gynecological disorders
- intestinal adhesions
- constipation
[Top]
How is appendicitis diagnosed?
A doctor or other health care provider can diagnose most cases of appendicitis by taking a person’s medical history and performing a physical examination. If a person shows classic symptoms, a doctor may suggest surgery right away to remove the appendix before it bursts. Doctors may use laboratory and imaging tests to confirm appendicitis if a person does not have classic symptoms. Tests may also help diagnose appendicitis in people who cannot adequately describe their symptoms, such as children or the mentally impaired.
Medical History
The doctor will ask specific questions about symptoms and health history. Answers to these questions will help rule out other conditions. The doctor will want to know when the pain began and its exact location and severity. Knowing when other symptoms appeared relative to the pain is also helpful. The doctor will ask questions about other medical conditions, previous illnesses and surgeries, and use of medications, alcohol, or illegal drugs.
Physical Examination
Details about the abdominal pain are key to diagnosing appendicitis. The doctor will assess pain by touching or applying pressure to specific areas of the abdomen.
Responses that may indicate appendicitis include
Guarding. Guarding occurs when a person subconsciously tenses the abdominal muscles during an examination. Voluntary guarding occurs the moment the doctor’s hand touches the abdomen. Involuntary guarding occurs before the doctor actually makes contact.
Rebound tenderness. A doctor tests for rebound tenderness by applying hand pressure to a patient’s abdomen and then letting go. Pain felt upon the release of the pressure indicates rebound tenderness. A person may also experience rebound tenderness as pain when the abdomen is jarred—for example, when a person bumps into something or goes over a bump in a car.
Rovsing’s sign. A doctor tests for Rovsing’s sign by applying hand pressure to the lower left side of the abdomen. Pain felt on the lower right side of the abdomen upon the release of pressure on the left side indicates the presence of Rovsing’s sign.
Psoas sign. The right psoas muscle runs over the pelvis near the appendix. Flexing this muscle will cause abdominal pain if the appendix is inflamed. A doctor can check for the psoas sign by applying resistance to the right knee as the patient tries to lift the right thigh while lying down.
Obturator sign. The right obturator muscle also runs near the appendix. A doctor tests for the obturator sign by asking the patient to lie down with the right leg bent at the knee. Moving the bent knee left and right requires flexing the obturator muscle and will cause abdominal pain if the appendix is inflamed.
Women of childbearing age may be asked to undergo a pelvic exam to rule out gynecological conditions, which sometimes cause abdominal pain similar to appendicitis.
The doctor may also examine the rectum, which can be tender from appendicitis.
Laboratory Tests
Blood tests are used to check for signs of infection, such as a high white blood cell count. Blood tests may also show dehydration or fluid and electrolyte imbalances. Urinalysis is used to rule out a urinary tract infection. Doctors may also order a pregnancy test for women.
Imaging Tests
Computerized tomography (CT) scans, which create cross-sectional images of the body, can help diagnose appendicitis and other sources of abdominal pain. Ultrasound is sometimes used to look for signs of appendicitis, especially in people who are thin or young. An abdominal x ray is rarely helpful in diagnosing appendicitis but can be used to look for other sources of abdominal pain. Women of childbearing age should have a pregnancy test before undergoing x rays or CT scanning. Both use radiation and can be harmful to a developing fetus. Ultrasound does not use radiation and is not harmful to a fetus.
[Top]
How is appendicitis treated?
Surgery
Typically, appendicitis is treated by removing the appendix. If appendicitis is suspected, a doctor will often suggest surgery without conducting extensive diagnostic testing. Prompt surgery decreases the likelihood the appendix will burst.
Surgery to remove the appendix is called appendectomy and can be done two ways. The older method, called laparotomy, removes the appendix through a single incision in the lower right area of the abdomen. The newer method, called laparoscopic surgery, uses several smaller incisions and special surgical tools fed through the incisions to remove the appendix. Laparoscopic surgery leads to fewer complications, such as hospital-related infections, and has a shorter recovery time.
Surgery occasionally reveals a normal appendix. In such cases, many surgeons will remove the healthy appendix to eliminate the future possibility of appendicitis. Occasionally, surgery reveals a different problem, which may also be corrected during surgery.
Sometimes an abscess forms around a burst appendix—called an appendiceal abscess. An abscess is a pus-filled mass that results from the body’s attempt to keep an infection from spreading. An abscess may be addressed during surgery or, more commonly, drained before surgery. To drain an abscess, a tube is placed in the abscess through the abdominal wall. CT is used to help find the abscess. The drainage tube is left in place for about 2 weeks while antibiotics are given to treat infection. Six to 8 weeks later, when infection and inflammation are under control, surgery is performed to remove what remains of the burst appendix.
Nonsurgical Treatment
Nonsurgical treatment may be used if surgery is not available, if a person is not well enough to undergo surgery, or if the diagnosis is unclear. Some research suggests that appendicitis can get better without surgery. Nonsurgical treatment includes antibiotics to treat infection and a liquid or soft diet until the infection subsides. A soft diet is low in fiber and easily breaks down in the gastrointestinal tract.
Recovery
With adequate care, most people recover from appendicitis and do not need to make changes to diet, exercise, or lifestyle. Full recovery from surgery takes about 4 to 6 weeks. Limiting physical activity during this time allows tissues to heal.
[Top]
What should people do if they think they have appendicitis?
Appendicitis is a medical emergency that requires immediate care. People who think they have appendicitis should see a doctor or go to the emergency room right away. Swift diagnosis and treatment reduce the chances the appendix will burst and improve recovery time.
[Top]
Points to Remember
- Appendicitis is a painful swelling and infection of the appendix.
- The appendix is a fingerlike pouch attached to the large intestine and located in the lower right area of the abdomen.
- Symptoms of appendicitis may include abdominal pain, loss of appetite, nausea, vomiting, constipation or diarrhea, inability to pass gas, low-grade fever, and abdominal swelling.
- A doctor can diagnose most cases of appendicitis by taking a person’s medical history and performing a physical examination. Sometimes laboratory and imaging tests are needed to confirm the diagnosis.
- Appendicitis is typically treated by removing the appendix.
- Appendicitis is a medical emergency that requires immediate care.
Jump to: navigation, search
Tuberculosis
Classification and external resources
Chest X-ray of a patient suffering from tuberculosis
ICD-10
A15.-A19.
ICD-9
010-018
OMIM
607948
DiseasesDB
8515
MedlinePlus
000077 000624
eMedicine
med/2324 emerg/618 radio/411
MeSH
D014376
Tuberculosis (abbreviated as TB for tubercle bacillus or Tuberculosis) is a common and often deadly infectious disease caused by mycobacteria, in humans mainly Mycobacterium tuberculosis. Tuberculosis usually attacks the lungs (as pulmonary TB) but can also affect the central nervous system, the lymphatic system, the circulatory system, the genitourinary system, the gastrointestinal system, bones, joints, and even the skin. Other mycobacteria such as Mycobacterium bovis, Mycobacterium africanum, Mycobacterium canetti, and Mycobacterium microti also cause tuberculosis, but these species are less common in humans.
The classic symptoms of tuberculosis are a chronic cough with blood-tinged sputum, fever, night sweats, and weight loss. Infection of other organs causes a wide range of symptoms. The diagnosis relies on radiology (commonly chest X-rays), a tuberculin skin test, blood tests, as well as microscopic examination and microbiological culture of bodily fluids. Tuberculosis treatment is difficult and requires long courses of multiple antibiotics. Contacts are also screened and treated if necessary. Antibiotic resistance is a growing problem in (extensively) multi-drug-resistant tuberculosis. Prevention relies on screening programs and vaccination, usually with Bacillus Calmette-Guérin (BCG vaccine).
Tuberculosis is spread through the air, when people who have the disease cough, sneeze, or spit. One–third of the world's current population has been infected with M. tuberculosis, and new infections occur at a rate of one per second However, most of these cases will not develop the full-blown disease; asymptomatic, latent infection is most common. About one in ten of these latent infections will eventually progress to active disease, which, if left untreated, kills more than half of its victims. The proportion of people in the general population who become sick with tuberculosis each year is stable or falling worldwide but, because of population growth, the absolute number of new cases is still increasing In 2004, mortality and morbidity statistics included 14.6 million chronic active cases, 8.9 million new cases, and 1.6 million deaths, mostly in developing countries In addition, a rising number of people in the developed world are contracting tuberculosis because their immune systems are compromised by immunosuppressive drugs, substance abuse, or AIDS. The distribution of tuberculosis is not uniform across the globe with about 80% of the population in many Asian and African countries testing positive in tuberculin tests, while only 5-10% of the US population test positive.It is estimated that the US has 25,000 new cases of tuberculosis each year, 40% of which occur in immigrants from countries where tuberculosis is endemic.
Classification
Main article: Tuberculosis classification
The current clinical classification system for tuberculosis (TB) is based on the pathogenesis of the disease.[citation needed]
Classification System for TB
Class
Type
Description
0
No TB exposureNot infected
No history of exposureNegative reaction to tuberculin skin test
1
TB exposureNo evidence of infection
History of exposureNegative reaction to tuberculin skin test
2
TB infectionNo disease
Positive reaction to tuberculin skin testNegative bacteriologic studies (if done)No clinical, bacteriologic, or radiographic evidence of TB
3
TB, clinically active
M. tuberculosis cultured (if done)Clinical, bacteriologic, or radiographic evidence of current disease
4
TBNot clinically active
History of episode(s) of TBorAbnormal but stable radiographic findingsPositive reaction to the tuberculin skin testNegative bacteriologic studies (if done)andNo clinical or radiographic evidence of current disease
5
TB suspect
Diagnosis pendingTB disease should be ruled in or out within 3 months
Signs and symptoms
Main symptoms of variants and stages of tuberculosis, with many symptoms overlapping with other variants, while others are more (but not entirely) specific for certain variants. Multiple variants may be present simultaneously.
When the disease becomes active, 75% of the cases are pulmonary TB. Symptoms include chest pain, coughing up blood, and a productive, prolonged cough for more than three weeks. Systemic symptoms include fever, chills, night sweats, appetite loss, weight loss, pallor, and often a tendency to fatigue very easily.
In the other 25% of active cases, the infection moves from the lungs, causing other kinds of TB, collectively denoted extrapulmonary tuberculosis. This occurs more commonly in immunosuppressed persons and young children. Extrapulmonary infection sites include the pleura in tuberculosis pleurisy, the central nervous system in meningitis, the lymphatic system in scrofula of the neck, the genitourinary system in urogenital tuberculosis, and bones and joints in Pott's disease of the spine. An especially serious form is disseminated TB, more commonly known as miliary tuberculosis. Although extrapulmonary TB is not contagious, it may co-exist with pulmonary TB, which is contagious
Causes
Main article: Mycobacterium tuberculosis
Scanning electron micrograph of Mycobacterium tuberculosis
The primary cause of TB, Mycobacterium tuberculosis, is an aerobic bacterium that divides every 16 to 20 hours, an extremely slow rate compared with other bacteria, which usually divide in less than an hour (For example, one of the fastest-growing bacteria is a strain of E. coli that can divide roughly every 20 minutes.) Since MTB has a cell wall but lacks a phospholipid outer membrane, it is classified as a Gram-positive bacterium. However, if a Gram stain is performed, MTB either stains very weakly Gram-positive or does not retain dye due to the high lipid & mycolic acid content of its cell wall MTB is a small rod-like bacillus that can withstand weak disinfectants and survive in a dry state for weeks. In nature, the bacterium can grow only within the cells of a host organism, but M. tuberculosis can be cultured in vitro.
Using histological stains on expectorate samples from phlegm (also called sputum), scientists can identify MTB under a regular microscope. Since MTB retains certain stains after being treated with acidic solution, it is classified as an acid-fast bacillus (AFB). The most common acid-fast staining technique, the Ziehl-Neelsen stain, dyes AFBs a bright red that stands out clearly against a blue background. Other ways to visualize AFBs include an auramine-rhodamine stain and fluorescent microscopy.
Phylogenetic tree of the genus Mycobacterium.
The M. tuberculosis complex includes three other TB-causing mycobacteria: M. bovis, M. africanum and M. microti. M. africanum is not widespread, but in parts of Africa it is a significant cause of tuberculosis. M. bovis was once a common cause of tuberculosis, but the introduction of pasteurized milk has largely eliminated this as a public health problem in developed countries. M. microti is mostly seen in immunodeficient people, although it is possible that the prevalence of this pathogen has been underestimated.
Other known pathogenic mycobacteria include Mycobacterium leprae, Mycobacterium avium and M. kansasii. The last two are part of the nontuberculous mycobacteria (NTM) group. Nontuberculous mycobacteria cause neither TB nor leprosy, but they do cause pulmonary diseases resembling TB.
Risk factors
Twin studies in the 1940s showed that susceptibility to TB was heritable. If one of a pair of twins got TB, then and the other was more likely to get TB if he was identical than if he was not. Since then, specific gene polymorphisms in IL12B have been linked to tuberculosis susceptibility.
Patients with diabetes mellitus are at increased risk of contracting tuberculosis, and they have a poorer response to treatment, possibly due to poorer drug absorption
Other conditions that increase risk include IV drug abuse; recent TB infection or a history of inadequately treated TB; chest X-ray suggestive of previous TB, showing fibrotic lesions and nodules; silicosis; prolonged corticosteroid therapy and other immunosuppressive therapy; head and neck cancers; hematologic and reticuloendothelial diseases, such as leukemia and Hodgkin's disease; end-stage kidney disease; intestinal bypass or gastrectomy; chronic malabsorption syndromes; vitamin D deficiency; and low body weight.
Some drugs, including rheumatoid arthritis drugs that work by blocking tumor necrosis factor-alpha (an inflammation-causing cytokine), raise the risk of activating a latent infection due to the importance of this cytokine in the immune defense against TB.
Mechanism
Transmission
Further information: Transmission (medicine)
When people suffering from active pulmonary TB cough, sneeze, speak, or spit, they expel infectious aerosol droplets 0.5 to 5 µm in diameter. A single sneeze can release up to 40,000 droplets.Each one of these droplets may transmit the disease, since the infectious dose of tuberculosis is very low and the inhalation of just a single bacterium can cause a new infection.
People with prolonged, frequent, or intense contact are at particularly high risk of becoming infected, with an estimated 22% infection rate. A person with active but untreated tuberculosis can infect 10–15 other people per year Others at risk include people in areas where TB is common, people who inject drugs using unsanitary needles, residents and employees of high-risk congregate settings, medically under-served and low-income populations, high-risk racial or ethnic minority populations, children exposed to adults in high-risk categories, patients immunocompromised by conditions such as HIV/AIDS, people who take immunosuppressant drugs, and health care workers serving these high-risk clients.
Transmission can only occur from people with active — not latent — TB . The probability of transmission from one person to another depends upon the number of infectious droplets expelled by a carrier, the effectiveness of ventilation, the duration of exposure, and the virulence of the M. tuberculosis strain.The chain of transmission can, therefore, be broken by isolating patients with active disease and starting effective anti-tuberculous therapy. After two weeks of such treatment, people with non-resistant active TB generally cease to be contagious. If someone does become infected, then it will take at least 21 days, or three to four weeks, before the newly infected person can transmit the disease to others. TB can also be transmitted by eating meat infected with TB. Mycobacterium bovis causes TB in cattle. (See details below.)
Pathogenesis
About 90% of those infected with Mycobacterium tuberculosis have asymptomatic, latent TB infection (sometimes called LTBI), with only a 10% lifetime chance that a latent infection will progress to TB disease. However, if untreated, the death rate for these active TB cases is more than 50%..
TB infection begins when the mycobacteria reach the pulmonary alveoli, where they invade and replicate within the endosomes of alveolar macrophages. The primary site of infection in the lungs is called the Ghon focus, and is generally located in either the upper part of the lower lobe, or the lower part of the upper lobe. Bacteria are picked up by dendritic cells, which do not allow replication, although these cells can transport the bacilli to local (mediastinal) lymph nodes. Further spread is through the bloodstream to other tissues and organs where secondary TB lesions can develop in other parts of the lung (particularly the apex of the upper lobes), peripheral lymph nodes, kidneys, brain, and bone. All parts of the body can be affected by the disease, though it rarely affects the heart, skeletal muscles, pancreas and thyroid.[29]
Tuberculosis is classified as one of the granulomatous inflammatory conditions. Macrophages, T lymphocytes, B lymphocytes and fibroblasts are among the cells that aggregate to form a granuloma, with lymphocytes surrounding the infected macrophages. The granuloma functions not only to prevent dissemination of the mycobacteria, but also provides a local environment for communication of cells of the immune system. Within the granuloma, T lymphocytes (CD8+) secrete cytokines such as interferon gamma, which activates macrophages to destroy the bacteria with which they are infected. T lymphocytes (CD4+) can also directly kill infected cells.
Importantly, bacteria are not always eliminated within the granuloma, but can become dormant, resulting in a latent infection.Another feature of the granulomas of human tuberculosis is the development of cell death, also called necrosis, in the center of tubercles. To the naked eye this has the texture of soft white cheese and was termed caseous necrosis.
If TB bacteria gain entry to the bloodstream from an area of damaged tissue they spread through the body and set up many foci of infection, all appearing as tiny white tubercles in the tissues. This severe form of TB disease is most common in infants and the elderly and is called miliary tuberculosis. Patients with this disseminated TB have a fatality rate of approximately 20%, even with intensive treatment.
In many patients the infection waxes and wanes. Tissue destruction and necrosis are balanced by healing and fibrosis.Affected tissue is replaced by scarring and cavities filled with cheese-like white necrotic material. During active disease, some of these cavities are joined to the air passages bronchi and this material can be coughed up. It contains living bacteria and can therefore pass on infection. Treatment with appropriate antibiotics kills bacteria and allows healing to take place. Upon cure, affected areas are eventually replaced by scar tissue.
Diagnosis
For more details on this topic, see Tuberculosis diagnosis.
Mycobacterium tuberculosis (stained red) in sputum
Tuberculosis is diagnosed definitively by identifying the causative organism (Mycobacterium tuberculosis) in a clinical sample (for example, sputum or pus). When this is not possible, a probable diagnosis may be made using imaging (X-rays or scans) and/or a tuberculin skin test.
The main problem with tuberculosis diagnosis is the difficulty in culturing this slow-growing organism in the laboratory (it may take 4 to 12 weeks for blood or sputum culture). A complete medical evaluation for TB must include a medical history, a physical examination, a chest X-ray, microbiological smears and cultures. It may also include a tuberculin skin test, a serological test. The interpretation of the tuberculin skin test depends upon the person's risk factors for infection and progression to TB disease, such as exposure to other cases of TB or immunosuppression.
Currently, latent infection is diagnosed in a non-immunized person by a tuberculin skin test, which yields a delayed hypersensitivity type response to an extract made from M. tuberculosis.[Those immunized for TB or with past-cleared infection will respond with delayed hypersensitivity parallel to those currently in a state of infection, so the test must be used with caution, particularly with regard to persons from countries where TB immunization is common. Tuberculin tests have the disadvantage in that they may produce false negatives, especially when the patient is co-morbid with sarcoidosis, Hodgkins lymphoma, malnutrition, or most notably active tuberculosis disease.New TB tests are being developed that offer the hope of cheap, fast and more accurate TB testing. These include polymerase chain reaction detection of bacterial DNA, and assays to detect the release of interferon gamma in response to mycobacterial proteins such as ESAT-6. These are not affected by immunization or environmental mycobacteria, so generate fewer false positive results. The development of a rapid and inexpensive diagnostic test would be particularly valuable in the developing world.
Prevention
TB prevention and control takes two parallel approaches. In the first, people with TB and their contacts are identified and then treated. Identification of infections often involves testing high-risk groups for TB. In the second approach, children are vaccinated to protect them from TB. Unfortunately, no vaccine is available that provides reliable protection for adults. However, in tropical areas where the levels of other species of mycobacteria are high, exposure to nontuberculous mycobacteria gives some protection against TB.
The World Health Organization (W.H.O.) declared TB a global health emergency in 1993, and the Stop TB Partnership developed a Global Plan to Stop Tuberculosis that aims to save 14 million lives between 2006 and 2015.Since humans are the only host of Mycobacterium tuberculosis, eradication would be possible: a goal that would be helped greatly by an effective vaccine.
Vaccines
Many countries use Bacillus Calmette-Guérin (BCG) vaccine as part of their TB control programs, especially for infants. According to the W.H.O., this is the most often used vaccine worldwide, with 85% of infants in 172 countries immunized in 1993.This was the first vaccine for TB and developed at the Pasteur Institute in France between 1905 and 1921. However, mass vaccination with BCG did not start until after World War II. The protective efficacy of BCG for preventing serious forms of TB (e.g. meningitis) in children is greater than 80%; its protective efficacy for preventing pulmonary TB in adolescents and adults is variable, ranging from 0 to 80%.
In South Africa, the country with the highest prevalence of TB, BCG is given to all children under age three. However, BCG is less effective in areas where mycobacteria are less prevalent; therefore BCG is not given to the entire population in these countries. In the USA, for example, BCG vaccine is not recommended except for people who meet specific criteria:
Infants or children with negative skin test results who are continually exposed to untreated or ineffectively treated patients or will be continually exposed to multidrug-resistant TB.
Healthcare workers considered on an individual basis in settings in which a high percentage of MDR-TB patients has been found, transmission of MDR-TB is likely, and TB control precautions have been implemented and were not successful.
BCG provides some protection against severe forms of pediatric TB, but has been shown to be unreliable against adult pulmonary TB, which accounts for most of the disease burden worldwide. Currently, there are more cases of TB on the planet than at any other time in history and most agree there is an urgent need for a newer, more effective vaccine that would prevent all forms of TB—including drug resistant strains—in all age groups and among people with HIV.
Several new vaccines to prevent TB infection are being developed. The first recombinant tuberculosis vaccine rBCG30, entered clinical trials in the United States in 2004, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID).[A 2005 study showed that a DNA TB vaccine given with conventional chemotherapy can accelerate the disappearance of bacteria as well as protect against re-infection in mice; it may take four to five years to be available in humans. A very promising TB vaccine, MVA85A, is currently in phase II trials in South Africa by a group led by Oxford University, and is based on a genetically modified vaccinia virus. Many other strategies are also being used to develop novel vaccines, including both subunit vaccines (fusion molecules composed of two recombinant proteins delivered in an adjuvant) such as Hybrid-1, HyVac4 or M72, and recombinant adenoviruses such as Ad35. Some of these vaccines can be effectively administered without needles, making them preferable for areas where HIV is very common. All of these vaccines have been successfully tested in humans and are now in extended testing in TB-endemic regions. In order to encourage further discovery, researchers and policymakers are promoting new economic models of vaccine development including prizes, tax incentives and advance market commitments.
The Bill and Melinda Gates Foundation has been a strong supporter of new TB vaccine development. Most recently, they announced a $200 million grant to the Aeras Global TB Vaccine Foundation for clinical trials on up to six different TB vaccine candidates currently in the pipeline.
Screening
Please help improve this article or section by expanding it. Further information might be found on the talk page. (June 2009)
Mantoux tuberculin skin test
Mantoux tuberculin skin tests are often used for routine screening of high risk individuals.
Chest photofluorography has been used in the past for mass screening for tuberculosis.
Treatment
Main article: Tuberculosis treatment
Treatment for TB uses antibiotics to kill the bacteria. The two antibiotics most commonly used are rifampicin and isoniazid. However, instead of the short course of antibiotics typically used to cure other bacterial infections, TB requires much longer periods of treatment (around 6 to 12 months) to entirely eliminate mycobacteria from the body. Latent TB treatment usually uses a single antibiotic, while active TB disease is best treated with combinations of several antibiotics, to reduce the risk of the bacteria developing antibiotic resistance. People with latent infections are treated to prevent them from progressing to active TB disease later in life. However, treatment using Rifampicin and Pyrazinamide is not risk-free. The Centers for Disease Control and Prevention (CDC) notified healthcare professionals of revised recommendations against the use of rifampin plus pyrazinamide for treatment of latent tuberculosis infection, due to high rates of hospitalization and death from liver injury associated with the combined use of these drugs.
Drug resistant tuberculosis is transmitted in the same way as regular TB. Primary resistance occurs in persons who are infected with a resistant strain of TB. A patient with fully susceptible TB develops secondary resistance (acquired resistance) during TB therapy because of inadequate treatment, not taking the prescribed regimen appropriately, or using low quality medication.Drug-resistant TB is a public health issue in many developing countries, as treatment is longer and requires more expensive drugs. Multi-drug-resistant tuberculosis (MDR-TB) is defined as resistance to the two most effective first-line TB drugs: rifampicin and isoniazid. Extensively drug-resistant TB (XDR-TB) is also resistant to three or more of the six classes of second-line drugs.The DOTS (Directly Observed Treatment Short-course) strategy of tuberculosis treatment based on clinical trials done in the 1970s by Tuberculosis Research Centre, Chennai, India, focusing on a neglected area of infectious disease control is now showing promising results in effectively treating all TB cases in the community.
Prognosis
Progression from TB infection to TB disease occurs when the TB bacilli overcome the immune system defenses and begin to multiply. In primary TB disease—1–5% of cases—this occurs soon after infection. However, in the majority of cases, a latent infection occurs that has no obvious symptoms. These dormant bacilli can produce tuberculosis in 2–23% of these latent cases, often many years after infection.The risk of reactivation increases with immunosuppression, such as that caused by infection with HIV. In patients co-infected with M. tuberculosis and HIV, the risk of reactivation increases to 10% per year.
Epidemiology
300, orange = 200–300, yellow = 100–200, green = 50–100, blue =
World TB incidence. Cases per 100,000; Red => 300, orange = 200–300, yellow = 100–200, green = 50–100, blue =< grey =" n/a." class="mw-redirect" title="WHO" href="http://en.wikipedia.org/wiki/WHO">WHO, 2006.
Annual number of new reported TB cases. Data from WHO.
According to the World Health Organization (WHO), nearly 2 billion people—one third of the world's population—have been exposed to the tuberculosis pathogen. Annually, 8 million people become ill with tuberculosis, and 2 million people die from the disease worldwide.In 2004, around 14.6 million people had active TB disease with 9 million new cases. The annual incidence rate varies from 356 per 100,000 in Africa to 41 per 100,000 in the Americas.Tuberculosis is the world's greatest infectious killer of women of reproductive age and the leading cause of death among people with HIV/AIDS.
The rise in HIV infections and the neglect of TB control programs have enabled a resurgence of tuberculosis.The emergence of drug-resistant strains has also contributed to this new epidemic with, from 2000 to 2004, 20% of TB cases being resistant to standard treatments and 2% resistant to second-line drugs. The rate at which new TB cases occur varies widely, even in neighboring countries, apparently because of differences in health care systems.
In 2005, the country with the highest estimated incidence of TB was Swaziland, with 1262 cases per 100,000 people. India has the largest number of infections, with over 1.8 million cases.In developed countries, tuberculosis is less common and is mainly an urban disease. In the United Kingdom, TB incidences range from 40 per 100,000 in London to less than 5 per 100,000 in the rural South West of England; the national average is 13 per 100,000. The highest rates in Western Europe are in Portugal (31.1 per 100,000 in 2005) and Spain (20 per 100,000). These rates compare with 113 per 100,000 in China and 64 per 100,000 in Brazil. In the United States, the overall tuberculosis case rate was 4.9 per 100,000 persons in 2004In Canada tuberculosis is still endemic in rural Manitoba
The incidence of TB varies with age. In Africa, TB primarily affects adolescents and young adults.However, in countries where TB has gone from high to low incidence, such as the United States, TB is mainly a disease of older people, or of the immunocompromised .
There are a number of known factors that make people more susceptible to TB infection: worldwide the most important of these is HIV. Co-infection with HIV is a particular problem in Sub-Saharan Africa, due to the high incidence of HIV in these countries.Smoking more than 20 cigarettes a day also increases the risk of TB by two to four times.Diabetes mellitus is also an important risk factor that is growing in importance in developing countries. Other disease states that increase the risk of developing tuberculosis are Hodgkin lymphoma, end-stage renal disease, chronic lung disease, malnutrition, and alcoholism.
Diet may also modulate risk. For example, among immigrants in London from the Indian subcontinent, vegetarian Hindu Asians were found to have an 8.5 fold increased risk of tuberculosis, compared to Muslims who ate meat and fish daily. Although a causal link is not proved by this data, this increased risk could be caused by micronutrient deficiencies: possibly iron, vitamin B12 or vitamin D.Further studies have provided more evidence of a link between vitamin D deficiency and an increased risk of contracting tuberculosis.Globally, the severe malnutrition common in parts of the developing world causes a large increase in the risk of developing active tuberculosis, due to its damaging effects on the immune system. Along with overcrowding, poor nutrition may contribute to the strong link observed between tuberculosis and poverty.
History
Tubercular decay has been found in the spines of Egyptian mummies. Pictured: Egyptian mummy in the British Museum
Tuberculosis has been present in humans since antiquity. The earliest unambiguous detection of Mycobacterium tuberculosis is in the remains of bison dated 18,000 years before the present. Whether tuberculosis originated in cattle and then transferred to humans, or diverged from a common ancestor infecting a different species, is currently unclear.However, it is clear that M. tuberculosis is not directly descended from M. bovis, which seems to have evolved relatively recently.
Skeletal remains show prehistoric humans (7000 BC) had TB , and tubercular decay has been found in the spines of mummies from 3000–2400 BC.Phthisis is a Greek term for tuberculosis; around 460 BC, Hippocrates identified phthisis as the most widespread disease of the times involving coughing up blood and fever, which was almost always fatal.In South America, the earliest evidence of tuberculosis is associated with the Paracas-Caverna culture (circa 750 BC to circa 100 AD).
Other names
Dr. Robert Koch discovered the tuberculosis bacilli.
In the past, tuberculosis has been called consumption, because it seemed to consume people from within, with a bloody cough, fever, pallor, and long relentless wasting. Other names included phthisis (Greek for consumption) and phthisis pulmonalis; scrofula (in adults), affecting the lymphatic system and resulting in swollen neck glands; tabes mesenterica, TB of the abdomen and lupus vulgaris, TB of the skin; wasting disease; white plague, because sufferers appear markedly pale; king's evil, because it was believed that a king's touch would heal scrofula; and Pott's disease, or gibbus of the spine and joints.Miliary tuberculosis—now commonly known as disseminated TB—occurs when the infection invades the circulatory system resulting in lesions which have the appearance of millet seeds on X-ray.TB is also called Koch's disease after the scientist Robert Koch.
Folklore
Before the Industrial Revolution, tuberculosis may sometimes have been regarded as vampirism. When one member of a family died from it, the other members that were infected would lose their health slowly. People believed that this was caused by the original victim draining the life from the other family members. Furthermore, people who had TB exhibited symptoms similar to what people considered to be vampire traits. People with TB often have symptoms such as red, swollen eyes (which also creates a sensitivity to bright light), pale skin, extremely low body heat, a weak heart and coughing blood, suggesting the idea that the only way for the afflicted to replenish this loss of blood was by sucking blood.Another folk belief attributed it to being forced, nightly, to attend fairy revels, so that the victim wasted away owing to lack of rest; this belief was most common when a strong connection was seen between the fairies and the dead.Similarly, but less commonly, it was attributed to the victims being "hagridden"—being transformed into horses by witches (hags) to travel to their nightly meetings, again resulting in a lack of rest.
TB was romanticized in the nineteenth century. Many people believed TB produced feelings of euphoria referred to as "Spes phthisica" or "hope of the consumptive". It was believed that TB sufferers who were artists had bursts of creativity as the disease progressed. It was also believed that TB sufferers acquired a final burst of energy just before they died which made women more beautiful and men more creative. In the early 20th century, some believed TB to be caused by masturbation.
Study and treatment
The study of tuberculosis, sometimes known as phthisiatry, dates back to The Canon of Medicine written by Ibn Sina (Avicenna) in the 1020s. He was the first physician to identify pulmonary tuberculosis as a contagious disease, the first to recognise the association with diabetes, and the first to suggest that it could spread through contact with soil and water. He developed the method of quarantine in order to limit the spread of tuberculosis. In ancient times, treatments focused on sufferers' diets. Pliny the Elder described several methods in his Natural History: "wolf's liver taken in thin wine, the lard of a sow that has been fed upon grass, or the flesh of a she-ass taken in broth".
Although it was established that the pulmonary form was associated with "tubercles" by Dr Richard Morton in 1689,[due to the variety of its symptoms, TB was not identified as a single disease until the 1820s and was not named "tuberculosis" until 1839 by J. L. Schönlein.During the years 1838 – 1845, Dr. John Croghan, the owner of Mammoth Cave, brought a number of tuberculosis sufferers into the cave in the hope of curing the disease with the constant temperature and purity of the cave air; they died within a year.The first TB sanatorium opened in 1854 in Görbersdorf, Germany (today Sokołowsko, Poland) by Hermann Brehmer.[
The bacillus causing tuberculosis, Mycobacterium tuberculosis, was identified and described on 24 March 1882 by Robert Koch. He received the Nobel Prize in physiology or medicine in 1905 for this discover Koch did not believe that bovine (cattle) and human tuberculosis were similar, which delayed the recognition of infected milk as a source of infection. Later, this source was eliminated by the pasteurization process. Koch announced a glycerine extract of the tubercle bacilli as a remedy for tuberculosis in 1890, calling it "tuberculin". It was not effective, but was later adapted as a test for pre-symptomatic tuberculosis.
The first genuine success in immunizing against tuberculosis was developed from attenuated bovine-strain tuberculosis by Albert Calmette and Camille Guérin in 1906. It was called "BCG" (Bacillus of Calmette and Guérin). The BCG vaccine was first used on humans in 1921 in France,[41] but it was not until after World War II that BCG received widespread acceptance in the USA, Great Britain, and Germany
Tuberculosis, or "consumption" as it was commonly known, caused the most widespread public concern in the 19th and early 20th centuries as an endemic disease of the urban poor In 1815, one in four deaths in England was of consumption; by 1918 one in six deaths in France were still caused by TB. In the 20th century, tuberculosis killed an estimated 100 million people. After the establishment in the 1880s that the disease was contagious, TB was made a notifiable disease in Britain; there were campaigns to stop spitting in public places, and the infected poor were pressured to enter sanatoria that resembled prisons; the sanatoria for the middle and upper classes offered excellent care and constant medical attention. Whatever the purported benefits of the fresh air and labor in the sanatoria, even under the best conditions, 50% of those who entered were dead within five years (1916).
Public health campaigns tried to halt the spread of TB
The promotion of Christmas Seals began in Denmark during 1904 as a way to raise money for tuberculosis programs. It expanded to the United States and Canada in 1907 – 1908 to help the National Tuberculosis Association (later called the American Lung Association).
In the United States, concern about the spread of tuberculosis played a role in the movement to prohibit public spitting except into spittoons.
In Europe, deaths from TB fell from 500 out of 100,000 in 1850 to 50 out of 100,000 by 1950. Improvements in public health were reducing tuberculosis even before the arrival of antibiotics, although the disease remained a significant threat to public health, such that when the Medical Research Council was formed in Britain in 1913 its initial focus was tuberculosis research.
It was not until 1946 with the development of the antibiotic streptomycin that effective treatment and cure became possible. Prior to the introduction of this drug, the only treatment besides sanatoria were surgical interventions, including the pneumothorax or plombage technique — collapsing an infected lung to "rest" it and allow lesions to heal — a technique that was of little benefit and was largely discontinued by the 1950s. The emergence of multidrug-resistant TB has again introduced surgery as part of the treatment for these infections. Here, surgical removal of chest cavities will reduce the number of bacteria in the lungs, as well as increasing the exposure of the remaining bacteria to drugs in the bloodstream, and is therefore thought to increase the effectiveness of the chemotherapy.
Hopes that the disease could be completely eliminated have been dashed since the rise of drug-resistant strains in the 1980s. For example, tuberculosis cases in Britain, numbering around 117,000 in 1913, had fallen to around 5,000 in 1987, but cases rose again, reaching 6,300 in 2000 and 7,600 cases in 2005. Due to the elimination of public health facilities in New York and the emergence of HIV, there was a resurgence in the late 1980s. The number of those failing to complete their course of drugs is high. New York had to cope with more than 20,000 TB patients with multidrug-resistant strains (resistant to, at least, both Rifampin and Isoniazid). The resurgence of tuberculosis resulted in the declaration of a global health emergency by the World Health Organization in 1993. Every year, nearly half a million new cases of multidrug-resistant tuberculosis (MDR-TB) are estimated to occur worldwide.
Evolution
Tuberculosis has co-evolved with humans for many thousands of years, and perhaps as much as several million years, but the oldest human remains showing signs of tuberculosis infection are 9,000 years old. During this evolution, M. tuberculosis has lost numerous coding and non-coding regions in its genome, losses that can be used to distinguish between strains of the bacteria. The implication is that M. tuberculosis strains differ geographically, so their genetic differences can be used to track the origins and movement of each strain.
Society and culture
See also: Tuberculosis in popular culture
Through its affecting important historical figures, tuberculosis has influenced particularly European history, and become a theme in art – mostly literature, music, and film.
Public health
Tuberculosis is one of the three primary diseases of poverty along with AIDS and malaria. The Global Fund to Fight AIDS, Tuberculosis and Malaria was started in 2002 to raise finances to address these infectious diseases. Globalization has lead to increased opportunities for disease spread. In 2007 a tuberculosis scare occurred when Andrew Speaker flew on a transatlantic flight well infected with multi-drug-resistant tuberculosis.
The National Center for HIV, STD, and TB Prevention as part of the Center for Disease Control and Prevention (CDC) is responsible for public health surveillance and prevention research in the United States.
The Mycobacterium Tuberculosis Structural Genomics Consortium is a global consortium of scientists conducting research regarding the diagnosis and treatment of tuberculosis. They are attempting to determine the 3-dimensional structures of proteins from M. Tuberculosis.[citation needed]
Infection of other animals
Main article: Mycobacterium bovis
Tuberculosis can be carried by mammals; domesticated species, such as cats and dogs, are generally free of tuberculosis, but wild animals may be carriers.
Mycobacterium bovis causes TB in cattle. An effort to eradicate bovine tuberculosis from the cattle and deer herds of New Zealand is underway. It has been found that herd infection is more likely in areas where infected natural reservoir such as Australian brush-tailed possums come into contact with domestic livestock at farm/bush borders. Controlling the vectors through possum eradication and monitoring the level of disease in livestock herds through regular surveillance are seen as a "two-pronged" approach to ridding New Zealand of the disease.
In the Republic of Ireland and the United Kingdom, badgers have been identified as one vector species for the transmission of bovine tuberculosis. As a result, governments have come under pressure from some quarters, primarily dairy farmers, to mount an active campaign of eradication of badgers in certain areas with the purpose of reducing the incidence of bovine TB. The effectiveness of culling on the incidence of TB in cattle is a contentious issue, with proponents and opponents citing their own studies to support their position. For instance, a study by an Independent Study Group on badger culling reported on 18 June 2007 that it was unlikely to be effective and would only make a “modest difference” to the spread of TB and that "badger culling cannot meaningfully contribute to the future control of cattle TB"; in contrast, another report concluded that this policy would have a significant impact. On July 4 2008, the UK government decided against a proposed random culling policy.
-------------------





No comments:
Post a Comment